Lindus Health
Founded Year
2021Stage
Grant - II | AliveTotal Raised
$79.7MRevenue
$0000Mosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
+118 points in the past 30 days
About Lindus Health
Lindus Health conducts end-to-end clinical trials for the life sciences sector, using a technology-driven approach to streamline the process. The company provides services including study design, patient recruitment, clinical data capture, monitoring, and project management, facilitated by a proprietary software platform and access to electronic health records. It serves the biotechnology and pharmaceutical industries. The company was founded in 2021 and is based in London, United Kingdom.
Loading...
Lindus Health's Product Videos

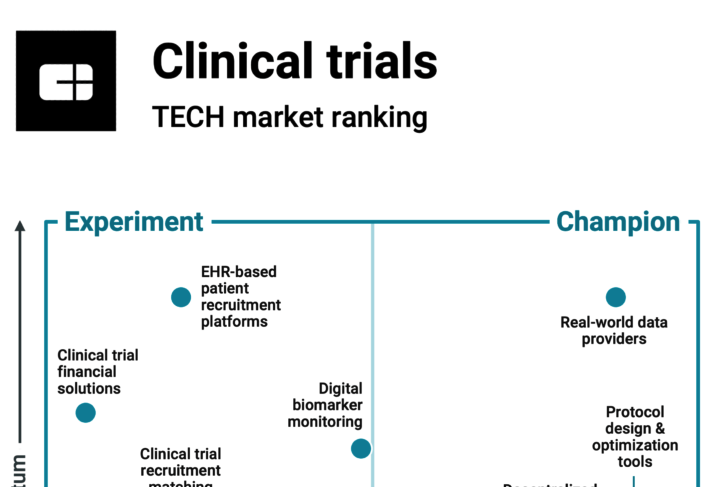
ESPs containing Lindus Health
The ESP matrix leverages data and analyst insight to identify and rank leading companies in a given technology landscape.
The clinical trial management systems market provides a range of software solutions that enable the management of clinical trials, including in protocol design, study planning, participant recruitment, data collection and analysis, and regulatory compliance. These systems can help streamline the clinical trial process, reduce administrative burden, and improve data quality and accuracy. Many of th…
Lindus Health named as Challenger among 15 other companies, including Oracle, Medidata, and Veeva Systems.
Lindus Health's Products & Differentiators
End to end clinical trials
Lindus Health is responsible for delivery of the entire clinical trial end to end. We deliver this using our world class clinical operations team and our unique technology platform (detailed features below)
Loading...
Research containing Lindus Health
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Lindus Health in 2 CB Insights research briefs, most recently on May 23, 2025.
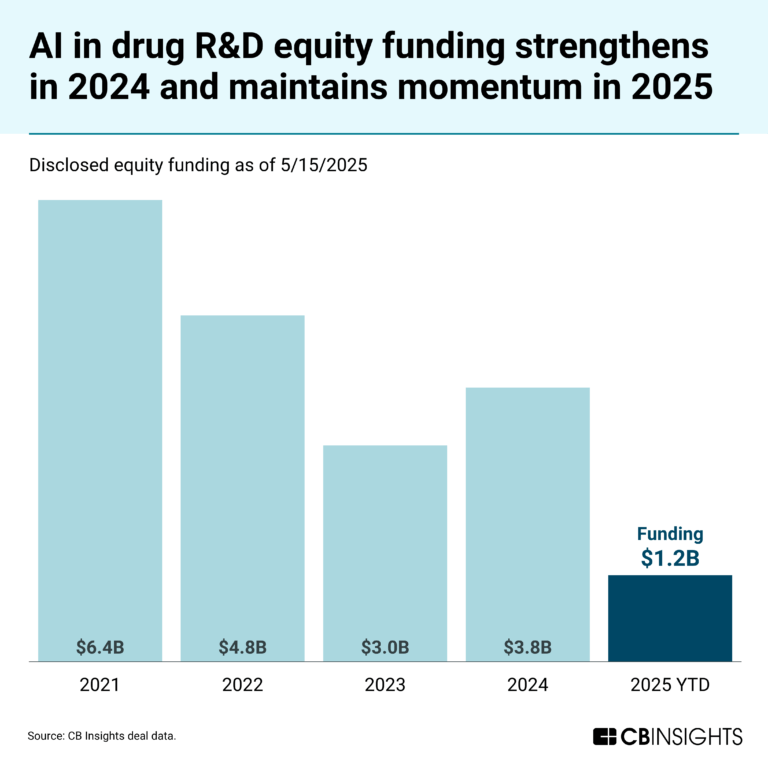
May 23, 2025
The AI in drug R&D market mapExpert Collections containing Lindus Health
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Lindus Health is included in 1 Expert Collection, including Digital Health.
Digital Health
11,422 items
The digital health collection includes vendors developing software, platforms, sensor & robotic hardware, health data infrastructure, and tech-enabled services in healthcare. The list excludes pureplay pharma/biopharma, sequencing instruments, gene editing, and assistive tech.
Latest Lindus Health News
Jul 31, 2025
| SkyQuest Technology Consulting News provided by Share this article Share toX The pharmaceutical industry's increasing focus on creating novel medications to treat a variety of chronic illnesses is the main factor propelling the expansion of the contract research organization sector. WESTFORD, Mass., July 31, 2025 /PRNewswire/ -- SkyQuest Technology Consulting published a report, titled, Contract Research Organization Market - Global Opportunity Analysis and Industry Forecast, 2025-2032", valued at USD 64.6 Billion in 2024. With a projected CAGR of 8.3% from 2025 to 2032, the market is expected to reach USD 122.2 Billion by the end of 2032. The increasing number of clinical trials conducted globally is the main factor propelling the growth of the contract research organization sector over the forecast period. Pharmaceutical companies' greater emphasis on research and development (R&D) activities to produce new products will be advantageous to the CRO sector. Globally, about one in three adults suffers from multiple chronic illnesses. The need for effective treatments is growing along with the number of chronic illnesses, which motivates pharmaceutical and biotechnology companies to develop new drugs. Download Sample Pages of the Report: https://www.skyquestt.com/sample-request/contract-research-organization-market Contract Research Organization Market Key Growth Drivers Over the years, pharmaceutical and biotechnology companies have increasingly used CROs in R&D due to their cost-effectiveness and efficiency. This would allow companies to leverage the specialized knowledge offered by other organizations while focusing their internal workforce or resources on core competencies. The need for effective solutions is being fuelled by demands for increasingly affordable products in the drug development industry. Moreover, two new growth opportunities in the contract research organization (CRO) market are the increasing demand for gene therapies and personalized medicine. These novel treatments would necessitate specialized clinical trials and regulatory knowledge, which might present CROs with chances to expand their product lines in the development of sophisticated therapies. Recent Developments in Contract Research Organization Market In March 2024, Heeds, a European contract research organization was acquired. Veeda Clinical Research Ltd., a leading CRO based in India was behind this acquisition as it focuses on expanding its business scope and operations to new regions. In July 2024, IQVIA, a well-known company in the global contract research organization landscape, announced the release of new technology to improve business operations. One Home for Sites brings together all of the systems at a clinical trial site into one, easy-to-use dashboard. In September 2024, A new contract research organization solution for ophthalmology clinical trials was launched by Lindus Health. The new solution can handle vast amounts of data generated from clinical trials to enhance the operation of contract research organizations compared to the traditional approach. Speak to our Analyst: https://www.skyquestt.com/speak-with-analyst/contract-research-organization-market Major Challenges in Contract Research Organization Industry Among the great challenges faced by clinical research organizations are the intricate and ever-changing regulatory environments in various countries; the number of rules and regulations associated with clinical trials and data management delays the setup of clinical trials, taking significant time and money away from other pursuits. For CROs, the regulatory challenges inhibit market growth and operational efficacy. Moreover, contract research organization operations are solely dependent on biotechnology and pharmaceutical companies. Any disruption to these companies can hinder contract research organizations market growth. The demand outlook for contract research organizations is also downgraded due to factors such as funding cuts and rejections from approvals. Competitive Landscape Major players in the contract research organization (CRO) market, including Quintiles IMS, Labcorp Drug Development, and Parexel are influencing the growth curve by providing comprehensive and innovative solutions at various stages of clinical trials, regulatory services, and data analytical services. These businesses, which are based on strategic partnerships, investments in state-of-the-art technology, performance efficiency initiatives, market accessibility, and standard shaping, all have extensive global reach that will improve their CRO service offerings. The major players in the Contract Research Organization industry include, ICON PLC Contract Research Organization Market Segmental Analysis The global contract research organization market is segmented into type, therapeutic area, end user, and region. By type, the market is classified into drug discovery, lead identification, lead optimization, pre-clinical, phase I trial services, phase II trial services, phase III trial services, and phase IV trial services. Depending on therapeutic area, it is divided into oncology, cardiology, infectious disease, neurology, immunological disorders, and gastroenterology & hepatology. According to end user, the market is bifurcated into pharmaceuticals & biotechnological companies and medical device companies. By type, the phase III trial services lead the market in 2024 with more late-stage clinical trials undertaken evaluating the efficacy and safety of a drug in large populations of patients. By therapeutic area, with the rising number of cancer patients across the globe and the increased number of clinical trials for immuno-oncology and targeted treatments, oncology led the market in 2024. By end user, the largest end user in 2024 is pharmaceutical & biotechnological companies who continued to outsource clinical research to CROs to reduce development costs and time to market. Contract Research Organization Market Full Report, Purchase Now (250+ Pages PDF with Insights, Charts, Tables, and Figures): https://www.skyquestt.com/buy-now/contract-research-organization-market Regional Outlook North America lead the contract research organization market, fueled by a large biotech and pharmaceutical industry, ample R&D spending, and a favorable regulatory environment for outsourcing clinical trials. Europe continued to make significant contributions, with a strong clinical research infrastructure and increasing collaboration between CROs and pharmaceutical companies in Germany, the UK, and France. Asia-Pacific grew the most rapidly due to low-cost trial services, fast recruitment, and high R&D spending in countries like China, India, and South Korea. LAMEA also steadily advanced, with increased CRO activity in Brazil and the United Arab Emirates because of regulatory modernization and the advancement of clinical trial networks. Explore Extensive ongoing Coverage on Healthcare Sector: Orthobiologics Market - Global Opportunity Analysis and Industry Forecast – 2032 https://www.skyquestt.com/report/orthobiologics-market Influenza Diagnostics Market - Global Opportunity Analysis and Industry Forecast – 2032 https://www.skyquestt.com/report/influenza-diagnostics-market Infectious Disease Diagnostics Market - Global Opportunity Analysis and Industry Forecast – 2032 https://www.skyquestt.com/report/infectious-disease-diagnostics-market Tissue Diagnostics Market - Global Opportunity Analysis and Industry Forecast – 2032 https://www.skyquestt.com/report/tissue-diagnostics-market Clinical Trials Market - Global Opportunity Analysis and Industry Forecast – 2032 https://www.skyquestt.com/report/clinical-trials-market About SkyQuest Technology Consulting SkyQuest Technology Consulting Group is a leading Business Consulting and Market Research firm, provides syndicated as well as customized research reports and consulting services. The company comprises a team of research analysts and consultants, adding more than 1200 market research reports to its database each year. These reports offer in-depth analysis on 40+ industries across 25 major countries worldwide, serving global clients across diverse industries. The company specializes in delivering customized intelligence, data-driven insights, and strategic advisory services that enable businesses to stay competitive and make informed decisions in rapidly evolving industries. Contact Us:
Lindus Health Frequently Asked Questions (FAQ)
When was Lindus Health founded?
Lindus Health was founded in 2021.
Where is Lindus Health's headquarters?
Lindus Health's headquarters is located at 90 Union Street, London.
What is Lindus Health's latest funding round?
Lindus Health's latest funding round is Grant - II.
How much did Lindus Health raise?
Lindus Health raised a total of $79.7M.
Who are the investors of Lindus Health?
Investors of Lindus Health include The Advanced Research and Invention Agency, Seedcamp, Creandum, Firstminute Capital, Visionaries Club and 21 more.
Who are Lindus Health's competitors?
Competitors of Lindus Health include Biorce, Current Health, Castor, Huma, Aparito and 7 more.
What products does Lindus Health offer?
Lindus Health's products include End to end clinical trials and 3 more.
Who are Lindus Health's customers?
Customers of Lindus Health include Habitual, Myota and DNA Nudge.
Loading...
Compare Lindus Health to Competitors

Medable is a company that offers a cloud-based platform for decentralized clinical trial technology in the healthcare and clinical research sectors. The platform facilitates electronic Clinical Outcome Assessments (eCOA), remote monitoring, and the integration of connected healthcare sensors for clinical trials. Medable was formerly known as Dermatrap. It was founded in 2012 and is based in Palo Alto, California.

ObvioHealth provides digital health solutions within the clinical trial industry. The company has a platform and mobile application allows for remote monitoring and participation in clinical trials, focusing on data collection and participant engagement. ObvioHealth's services are relevant to the healthcare sector, especially in trial management. It was founded in 2017 and is based in New York, New York.

Huma is a healthcare AI company that provides digital health solutions for care and research. The company offers services including remote patient monitoring, hospital-at-home programs, virtual wards, and decentralized clinical trials. Huma's technology is used by hospitals and clinics globally and is integrated into clinical trials. Huma was formerly known as Huma Therapeutics GmbH. It was founded in 2011 and is based in London, United Kingdom.

THREAD is a company focused on clinical research and electronic clinical outcome assessments (eCOA) within the life sciences sector. It offers a proprietary decentralized research platform and a suite of supporting services designed to enable remote data capture from participants and sites during clinical studies. It was founded in 2005 and is based in Tustin, California.

Curebase is a company that provides an eClinical platform in the clinical research sector, offering tools such as electronic patient-reported outcomes (ePRO), electronic clinical outcome assessments (eCOA), electronic consent (eConsent), and electronic data capture (EDC) systems. The platform is designed for study launches, data collection, and participant engagement. It was founded in 2017 and is based in San Francisco, California.

Evidation is involved in measuring health in everyday life within the digital health sector. The company provides a platform for individuals to track health activities, participate in research, and manage their data privacy. Evidation serves the life sciences, government, and non-profit organizations. It was founded in 2012 and is based in San Mateo, California.
Loading...