Enveda
Founded Year
2019Stage
Series D | AliveTotal Raised
$535.5MValuation
$0000Last Raised
$150M | 14 days agoMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
+14 points in the past 30 days
About Enveda
Enveda utilizes artificial intelligence (AI) to analyze natural molecules for drug discovery. The company provides a platform that identifies and characterizes molecules produced by living organisms, creating a database of chemical biodiversity. Enveda serves the pharmaceutical and life sciences sectors. Enveda was formerly known as Enveda Therapeutics. It was founded in 2019 and is based in Boulder, Colorado.
Loading...
Loading...
Research containing Enveda
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Enveda in 2 CB Insights research briefs, most recently on May 23, 2025.
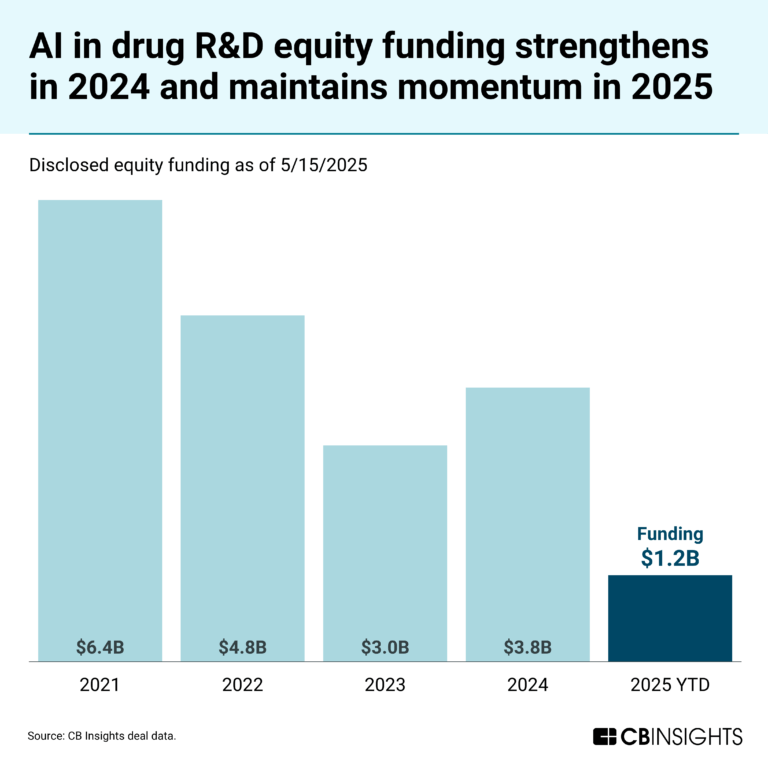
May 23, 2025
The AI in drug R&D market mapExpert Collections containing Enveda
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Enveda is included in 4 Expert Collections, including Digital Health.
Digital Health
11,440 items
The digital health collection includes vendors developing software, platforms, sensor & robotic hardware, health data infrastructure, and tech-enabled services in healthcare. The list excludes pureplay pharma/biopharma, sequencing instruments, gene editing, and assistive tech.
Drug Discovery Tech Market Map
221 items
This CB Insights Tech Market Map highlights 220 drug discovery companies that are addressing 12 distinct technology priorities that pharmaceutical companies face.
Artificial Intelligence
10,402 items
AI in drug discovery
524 items
Companies using AI to advance therapeutic discovery, categorized into: platforms (primary product is software) and discovery engines (primary product is therapeutics). Additional funnel descriptions reflect how companies are applying AI.
Latest Enveda News
Sep 16, 2025
Dr. Vince Clinical Research (DVCR), a full-service contract research organization (CRO) specializing in early phase and multi-site clinical trials, announces that the first subject has been dosed in a Phase 1b multi-site study evaluating ENV-294, Enveda's first-in-class anti-inflammatory drug candidate in adults with moderate to severe atopic dermatitis (AD). This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250916277343/en/ Dr. Vince Clinical Research, located at 7401 W. 91st Street, Overland Park, KS 66212. ENV-294 is a first-in-class small molecule developed using Enveda's proprietary drug discovery platform. It is designed to address atopic dermatitis and other chronic inflammatory diseases through a differentiated mechanism of action. ENV-294 demonstrates the potential to address unmet needs in moderate to severe AD patients who require systemic therapies but may not respond well to or tolerate currently available treatments. “DVCR has been an exceptional partner in supporting the advancement of our investigational drug, ENV-294, into the clinic,” said José Trevejo, MD, PhD, Chief Medical Officer and Head of Clinical Pipeline Strategy at Enveda. “Their deep operational expertise and collaborative approach were instrumental in the successful completion with promising data from our first-in-human study. We look forward to continuing this productive relationship as we progress through early clinical development.” This Phase 1b study follows a successful Phase 1a trial, also conducted by DVCR, which was a randomized, double-blind, placebo-controlled, single and multiple ascending dose study in healthy adults. ENV-294 was well-tolerated across all dose levels, with no dose-limiting toxicities or serious adverse events and demonstrated a favorable safety, tolerability and pharmacokinetic profile. The multi-site Phase 1b study is designed to build upon those results by assessing ENV-294's safety, tolerability, pharmacodynamics, target engagement and preliminary efficacy. “This important milestone, dosing the first subject with atopic dermatitis in the Phase 1b study, is a true testament to the scientific expertise and transformative work of the Enveda team,” said Dr. Brad Vince, CEO and Chief Medical Officer of DVCR. “We are honored that Enveda selected DVCR as a preferred partner for this important program.” According to the National Eczema Association, atopic dermatitis affects over 26 million Americans and approximately 200 million people worldwide. The condition is characterized by chronic, relapsing inflammation of the skin and is often associated with significant itch, skin pain and impaired quality of life. About Dr. Vince Clinical Research Dr. Vince Clinical Research (DVCR) is a full-service contract research organization (CRO) specializing in early phase trials in both healthy normal volunteers and patient populations across a wide range of trial designs and therapeutic areas such as neuroscience, substance abuse, pain, cardiometabolic disorders, infectious diseases and many others. CRO services include project management, data management, biostatistics, statistical programming, PK/PD analysis, clinical and medical monitoring, medical writing as well as site feasibility and management for multi-site trials with our global network of site partners. Additionally, DVCR operates one of the most innovative and technologically advanced clinical pharmacology units in the world with over 90 beds for overnight confinement, a cGMP compliant pharmacy as well as luxurious amenities to support diverse study participant recruitment and retention. By leveraging technology and one of the country's most experienced leadership teams in early clinical development, DVCR provides Smarter Faster Data ® to its biopharmaceutical clients. For more information, visit: https://drvince.com/cro Connect with DVCR on LinkedIn and YouTube View source version on businesswire.com: https://www.businesswire.com/news/home/20250916277343/en/ Contacts Media Contact: Laura Harding Vice President, Marketing Dr. Vince Clinical Research lharding@drvince.com
Enveda Frequently Asked Questions (FAQ)
When was Enveda founded?
Enveda was founded in 2019.
Where is Enveda's headquarters?
Enveda's headquarters is located at 5700 Flatiron Parkway, Boulder.
What is Enveda's latest funding round?
Enveda's latest funding round is Series D.
How much did Enveda raise?
Enveda raised a total of $535.5M.
Who are the investors of Enveda?
Investors of Enveda include FPV Ventures, Kinnevik, Lux Capital, Level Ventures, Lingotto Investment Management and 31 more.
Who are Enveda's competitors?
Competitors of Enveda include Brightseed and 5 more.
Loading...
Compare Enveda to Competitors

BioSymetrics integrates clinical and experimental data with machine learning to develop precision medicines. The company focuses on target discovery and precision medicine development, using clinical data and in vivo validation to find new drug targets. BioSymetrics serves sectors involved in neurological, cardiometabolic, and rare disease research and drug development. It was founded in 2015 and is based in Huntington, New York. In February 2025, BioSymetrics was acquired by Renovaro BioSciences.

Atomwise develops machine learning-based discovery engines and uses artificial intelligence (AI)-based neural networks to help discover new medicines. It predicts drug candidates for pharmaceutical companies, start-ups, and research institutions and designs drugs using computational drug design. It was formerly known as Chematria. The company was founded in 2012 and is based in San Francisco, California.

Nuritas engages in the discovery and development of bioactive peptides for various industries using artificial intelligence. Its offerings include peptide-based ingredients for dietary supplements, functional foods and beverages, medical foods, and cosmetics. The company serves the food and beverage, health and wellness, and cosmetics sectors with peptide solutions. It was founded in 2014 and is based in Dublin 2, Ireland.

PharmCADD is a company involved in drug discovery using technologies in the pharmaceutical sector. The company's main offerings include a platform that integrates artificial intelligence, molecular dynamics, and quantum mechanics for the design and discovery of drug candidates. PharmCADD also has an mRNA vaccine platform, PharmVAC, which focuses on treatment effects for patients. It was founded in 2019 and is based in Busan, South Korea.
ReviveMed works in the field of precision medicine with a focus on oncology and cardio-metabolic diseases, using artificial intelligence (AI) and metabolomics. The company has developed a metabolomic platform and digital metabolic twins that simulate disease progression and treatment response, utilizing various metabolites to aid in patient stratification and optimize clinical trials. The technology is designed to predict patient responses to therapies, specifically in relation to immuno-oncology and metabolic health. It was founded in 2016 and is based in Cambridge, Massachusetts.

BIOS Health focuses on neural engineering within the healthcare sector, developing infrastructure for future neural treatments. The company offers AI-powered neural interfaces and software that can interpret and modulate the body's neural signals to treat diseases. BIOS Health primarily targets the digital health treatments market, leveraging insights from the nervous system to create personalized medicine. BIOS Health was formerly known as Cambridge Bio-Augmentation Systems. It was founded in 2015 and is based in Cambridge, United Kingdom.
Loading...