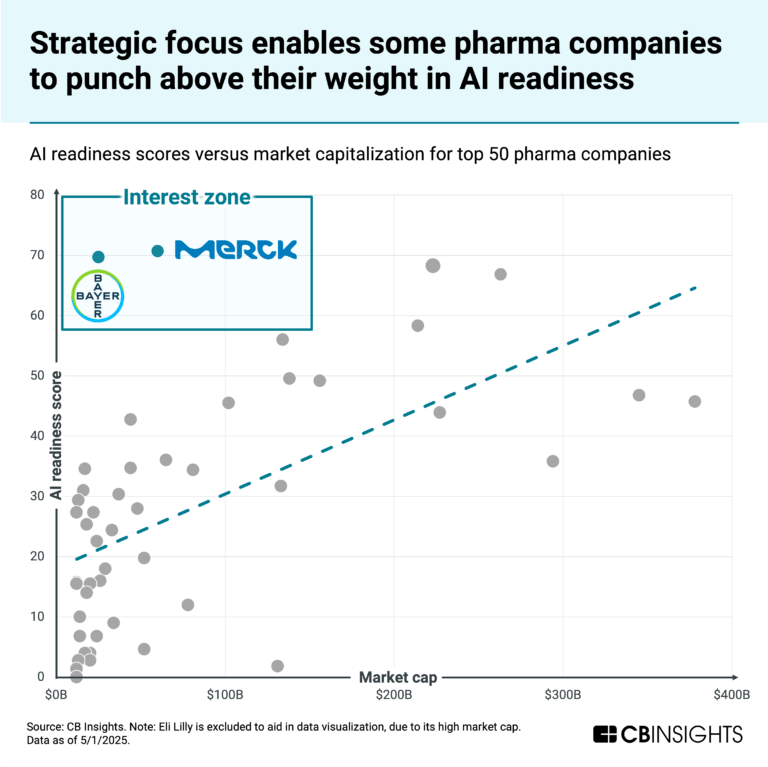
AbbVie
Founded Year
2013Stage
IPO | IPODate of IPO
1/2/2013Market Cap
374.26BStock Price
213.00Revenue
$0000About AbbVie
AbbVie operates as a biopharmaceutical company focused on developing medicines in the healthcare sector. The company works on therapeutics for various health issues, including immunology, oncology, neuroscience, eye care, and aesthetics. It was founded in 2013 and is based in North Chicago, Illinois.
Loading...
Loading...
Research containing AbbVie
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned AbbVie in 2 CB Insights research briefs, most recently on Jul 3, 2025.
Expert Collections containing AbbVie
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
AbbVie is included in 1 Expert Collection, including Fortune 500 Investor list.
Fortune 500 Investor list
590 items
This is a collection of investors named in the 2019 Fortune 500 list of companies. All CB Insights profiles for active investment arms of a Fortune 500 company are included.
AbbVie Patents
AbbVie has filed 1725 patents.
The 3 most popular patent topics include:
- clusters of differentiation
- monoclonal antibodies
- transcription factors
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
11/15/2019 | 3/18/2025 | Monoamine oxidase inhibitors, Prodrugs, Abandoned drugs, Phenols, Beta blockers | Grant |
Application Date | 11/15/2019 |
|---|---|
Grant Date | 3/18/2025 |
Title | |
Related Topics | Monoamine oxidase inhibitors, Prodrugs, Abandoned drugs, Phenols, Beta blockers |
Status | Grant |
Latest AbbVie News
Sep 5, 2025
CD47的一次罕见成功! 请有相关产品的biotech公司立即联系药时代BD团队(BD@drugtimes.cn)! 2025年8月28日,浙江昂利康制药股份有限公司(以下简称“昂利康”)发布公告,宣布其与亚飞(上海)生物医药科技有限公司(以下简称“亚飞生物”)、上海亲合力生物医药科技股份有限公司(以下简称“亲合力”)签署《战略合作协议之授权许可协议》,将获得靶向CD47的IMD-1005药物分子在中国(包括中华人民共和国大陆地区、香港特别行政区、澳门特别行政区以及中国台湾地区)的研发、生产、商业化独家权益。 根据协议,昂利康将分阶段向亚飞生物、亲合力合计支付1.5亿元人民币首付款,以及向亲合力支付最高不超过6.2亿元人民币的研发、销售里程碑付款。此外,在协议约定的销售分成期间,昂利康需向亲合力支付12.8%的销售分成。 近一两年,CD47研发领域整体并不乐观,失败案例数量远多于成功案例。昂利康的此次交易无疑为这一领域注入了新的活力。 为什么说CD47是块难啃的硬骨头 2009年,斯坦福大学医学院Irving Weissman 教授团队在 Cell 上发表了一篇题为“CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells“的论文。论文提到,经研究发现CD47在许多肿瘤细胞表面大量上调,通过使用抗CD47抗体,可以破坏CD47的“别吃我”信号,发出信号并释放巨噬细胞来吞噬肿瘤,在相关血液肿瘤中具有显著治疗作用。 在这篇论文结果的助推下,CD47受到业内广泛关注,并被业界寄予”下一个PD-1″的期望。但十几年来,药企在CD47领域的探索并不顺利,或者宣布失败,或者半路退出。 吉利德 2024年6月,吉利德在2024年欧洲血液学协会(EHA)会议上公布了其CD47单抗Magrolimab的III期 ENHANCE 研究新数据。数据显示,与安慰剂组相比,Magrolimab与化疗(阿扎胞苷+维奈克拉)的联用方案的完全缓解率更低,且死亡风险增加20.3%。也就是说,用了这款药,不仅病情得不到好转,还会增加死亡风险。 Magrolimab为吉利德49亿美元收购Forty Seven所得。2020年,通过这场价值不菲的收购,吉利德踌躇满志地入局CD47 ,跻身CD47领域头部玩家。但近4年的探索显示,CD47确实是个难啃的硬骨头。早在此项结果公布于众前,吉利德就已停止了这款CD47单抗的大部分临床试验的招募/研究(包括实体瘤和血液瘤)。 ENHANCE研究的停止意味着吉利德将全面放弃Magrolimab的开发。当时,吉利德表示,停止Magrolimab的研究是由于对该药物在实体瘤和血液肿瘤中所有可用的安全性和有效性数据进行了全面审查后的决定。 据悉,这是当时中国Biotech有史以来最大的跨国对外授权战略合作。 但就在合作达成2年后(2022年中),艾伯维接连宣布终止了lemzoparlimab的两项临床试验:1)lemzoparlimab与口服或静脉注射地塞米松制剂联用或不联用,治疗成人多发性骨髓瘤(MM)的研究;2)lemzoparlimab+阿扎胞苷+venetoclax 联合治疗骨髓增生异常综合征(MDS)/AML的 I 期临床试验。 在此基础上,2023年9月,艾伯维宣布终止了其与天境生物的这项合作,归还lemzoparlimab所有权益。 但就在完成其C轮融资十几个月后,Arch Oncology突然宣布终止其在研CD47管线的所有研发工作,并解散了公司大部分员工。 ALX的CD47项目也毫无意外的不顺利。2023年8月,ALX宣布将终止evorpacept的ASPEN-02和ASPEN-05研究,这两项研究分别用于评估evorpacept与化疗药物联合治疗MDS和AML的有效性。 辉瑞 目前,辉瑞的这两款CD47项目均已暂停。 CD47 还需更多成功案例 临床前研究结果显示,注射用IMD-1005在SHP77(人小细胞肺癌)及Raji(淋巴瘤)CDX肿瘤模型中,均能呈剂量依赖性抑制肿瘤生长,药效突出;在临床前动物安全性评价实验中,IMD-1005的安全性优秀。 安全性问题是目前CD47领域的重点关注方向,此前很多项目都是折在了药物的安全性问题上。这与CD47表达机制有关。前文提到,CD47在许多肿瘤细胞表面大量上调。此前,正是因为这种机制层面上展现出的适应症拓展潜力,才吸引了不少药企和投资公司来此掘金。但需要注意的是,CD47不仅在肿瘤细胞表面表达,同时还在人体红细胞高度表达。 当一款CD47抗体进入人体后,将优先和外周血中的红细胞和白细胞等正常细胞结合,未与正常细胞结合的 CD47抗体才能最终获得与肿瘤细胞结合的权力,起到肿瘤杀伤作用。因此,在研的 CD47管线一般剂量都不低。但这种高剂量会导致一个问题,即会有大量的CD47抗体与红细胞结合,并进一步导致红细胞被裂解/吞噬,最终导致溶血、贫血等不良反应发生。 因此,如何绕开CD47的高剂量血液毒性是一众CD47管线在开发中重点考量的问题。昂利康此次交易的重点——IMD-1005主要是通过技术手段优化抗体结构,从而提升安全性。该CD47抗体对 CD47 靶点的结合活性因偶联了遮罩而大大降低,在血液中高度稳定,从而避免了CD47抗体活性所导致的血液毒性。 目前CD47领域仍有大量类似IMD-1005的项目试图突破困境,改变该领域的研发现状。据智慧芽数据库信息显示,眼下全球仍有两百余条CD47管线在研,覆盖单抗、双抗、ADC、CAR-T等多个药物类型。其中,进入III期临床阶段的仅3家,分别为宜明昂科的timdarpacept、康方生物的ligufalimab及EpicentRx的nibrozetone。 国内方面,宜明昂科现在正在推进两项相关III期临床,分别为:1)timdarpacept与替雷利珠单抗联用治疗PD(L)-1单抗难治性经典型霍奇金淋巴瘤;2)timdarpacept联合阿扎胞苷治疗慢性粒-单核细胞白血病。(信息来源于第一财经文章) 康方生物针对ligufalimab的布局则涉及实体瘤及血液瘤两方面。其中,一项血液瘤临床研究是在美国开展的针对骨髓增生异常综合征的II期临床研究,一项实体瘤的临床研究是 CD47单抗联合 PD-1/VEGF双抗药物依沃西,对比K药一线治疗PD-L1表达阳性复发/转移性头颈部鳞状细胞癌的头对头III期临床试验。 此外,在ASCO2025上,ImmuneOncia Therapeutics公布了其新一代CD47抗体IMC-002联合仑伐替尼治疗晚期肝细胞癌(HCC)患者的1b期临床试验中期结果。在这项剂量扩展阶段研究,IMC-002展现出良好的安全性特征,未报告中性粒细胞减少症或血小板减少症病例。13例患者中有2例(15%)出现轻度贫血,96%的不良事件为1-2级,且主要发生在首个治疗周期。 目前,这些CD47产品均进展良好。这也说明一个问题,即:虽已有不少失败案例在前,但该领域仍有不少成功案例正在陆续披露。 不过,仍需注意的是,在0个管线成功获批上市的前提下,CD47赛道的低成功率将导致投资者对该领域保持谨慎态度。一笔交易带给整个赛道的活力是有限的,无论交易还临床数据,CD47赛道仍在等待更多成功案例! 接下来是一条会议宣传: 目前,药时代与肝病新药联盟应行业需求,决定于2025年11月21-22日在上海举办“第四届肝病新药联盟年会暨减重新药论坛”活动(线下)。活动有幸邀请到了多位行业资深专家,将聚焦肝病及相关领域的最新科研成果、临床实践和未来发展方向,来自医院、药企、CRO、投资机构等单位的专家、学者们将齐聚一堂,从不同的角度分享他们的行业洞见和宝贵经验。欢迎感兴趣的读者持续关注,药时代公众号将持续更新会议最新动态。 主办单位:肝病新药联盟、药时代
AbbVie Frequently Asked Questions (FAQ)
When was AbbVie founded?
AbbVie was founded in 2013.
Where is AbbVie's headquarters?
AbbVie's headquarters is located at 1 North Waukegan Road, North Chicago.
What is AbbVie's latest funding round?
AbbVie's latest funding round is IPO.
Who are the investors of AbbVie?
Investors of AbbVie include Abbott.
Who are AbbVie's competitors?
Competitors of AbbVie include Cerba HealthCare, AstraZeneca, Sanofi, Bayer, Merck and 7 more.
Loading...
Compare AbbVie to Competitors
Sumitomo Pharma is a biopharmaceutical company focused on developing therapeutic and scientific breakthroughs. The company's main offerings include the development of novel cancer therapeutics and a robust pipeline of treatments aimed at addressing unmet clinical needs in oncology. Sumitomo Pharma was formerly known as Sumitomo Dainippon Pharma. It was founded in 1897 and is based in Osaka, Japan.
Boehringer Ingelheim is involved in healthcare within the pharmaceutical and animal health sectors. The company conducts research and development of products for humans and animals. Boehringer Ingelheim serves the healthcare industry with an emphasis on pharmaceutical products. It was founded in 1885 and is based in Ingelheim am Rhein, Germany.

LifeArc operates as a self-financing medical research charity. The organization specializes in drug discovery, diagnostics development, antibody humanization, and intellectual property management, aiming to lab-based scientific discoveries into diagnostics, treatments, and cures. LifeArc collaborates with various sectors including the pharmaceutical industry, biotechnology sector, and academic institutions to develop and commercialize medical research. LifeArc was formerly known as MRC Technology. It was founded in 2000 and is based in London, United Kingdom.

Servier is a pharmaceutical company that engages in the research, development, and production of medications for various diseases, including targeted therapies for cancer, cardiovascular treatments, diabetes, and drugs for immuno-inflammatory and neuropsychiatric conditions. The company also produces generic drugs. It was founded in 1954 and is based in Suresnes, France.

Daiichi Sankyo France operates as a global pharmaceutical company with a focus on providing therapeutic solutions in oncology and cardiology. The company offers treatments for various diseases including hypertension, dyslipidemia, diabetes, acute coronary syndrome, venous thromboembolism, breast cancer, metastatic melanoma, inflammatory diseases, and pain. Daiichi Sankyo France primarily serves the healthcare sector, providing innovative medications and supporting services to patients and medical professionals. It was founded in 2003 and is based in Rueil-Malmaison, France.

Cerba HealthCare provides medical diagnostics within the healthcare sector. It offers diagnostic services including proximity biology, specialty biology, clinical trial biology, anatomical and cytological pathology, medical imaging, and preventive biology. These services are used for the diagnosis and management of diseases. It was founded in 1967 and is based in Issy-les-Moulineaux, France.
Loading...